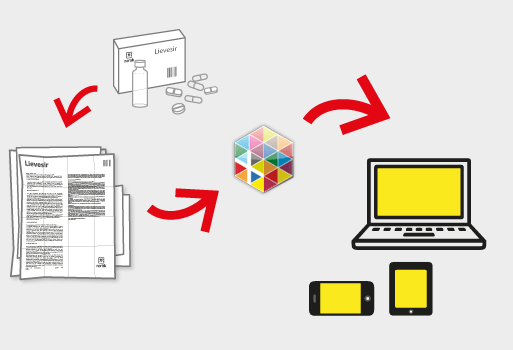
myHealthboxとは何ですか
myHealthboxは、消費者や専門家の両方のために自由に利用可能な、信頼できるデータを提供し、デジタル医療情報の最大の、独立して、ソースです。 myHealthboxに関係なく、デバイスや地域の企業に医療情報を簡単かつインタラクティブな共有のためのユニークなプラットフォームを提供します。
30億以上のインターネットユーザーの上昇、および健康関連情報のための一日あたり1.75億Google検索では、健康製品に、デジタルアクセス可能で、信頼できる情報に対する要求はこれまで以上に大きくなっています。私たちは、消費者や保健の専門家から、それらと係合したいこれらの企業のための、シンプルで質の高い情報の需要をカバーするためのシンプルなソリューションを提供します。
の検索エンジンは、従来の製薬薬から店頭や代替療法に、医療機器、局所クリームや栄養補助食品を、獣医学および植物製品に至るまでの健康関連商品の大きな選択のためのデジタル情報リーフレットを提供します。35の言語で利用できる、すでに200万以上のリーフレットでmyHealthboxは急速にその適用範囲およびユーザーベースを拡大しています。
出典
myHealthboxは、公式の情報源(保健省、薬局、販売承認保持者および生産者)からの完全に吟味され承認されたコンテンツおよび文書へのアクセスのみを提供します。
内容は変更されることなく提供され、関連する公式機関によって承認されています。
情報は定期的にチェックされ、更新されます(平均30日ごと)。

FDA
U.S. Food & Drud Administration
The Food and Drug Administration is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices; and by ensuring the safety of our nation's food supply, cosmetics, and products that emit radiation.

DailyMed
U.S. National Library of Medicine
DailyMed provides trustworthy information about marketed drugs. DailyMed is the official provider of FDA label information (package inserts). This Web site provides a standard, comprehensive, up-to-date, look-up and download resource of medication content and labeling found in medication package inserts.

BfArM
Federal Institute for Drugs and Medical Devices
The Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) is an independent federal higher authority within the portfolio of the Federal Ministry of Health.

DIMDI
German Institute for Medical Documentation and Information
The German Institute of Medical Documentation and Information (DIMDI), an authority within the German Federal Ministry of Health, provides high-quality information for all areas of the health system via Internet. It publishes official medical classifications and maintains medical terminologies, thesauri, nomenclatures and catalogues.

Digemid
Dirección General de Medicamentos, Insumos y Drogas
La Dirección General de Medicamentos Insumos y Drogas (DIGEMID) es un órgano de línea del Ministerio de Salud, creado con el Decreto Legislativo Nº 584 del 18 de Abril del año 1990. La DIGEMID es una institución técnico normativa que tiene como objetivo fundamental, lograr que la población tenga acceso a medicamentos seguros, eficaces y de calidad y que estos sean usados racionalmente.

SUKL
State Institute for Drug Control
Posláním Státního ústavu pro kontrolu léčiv je v zájmu ochrany zdraví občanů zajistit, aby v ČR byla dostupná pouze farmaceuticky jakostní, účinná a bezpečná humánní léčiva, a podílet se na tom, aby v ČR byly používány pouze bezpečné a funkční zdravotnické prostředky.

AMED
Agenţia Medicamentului şi Dispozitivelor Medicale
Agenţia Medicamentului şi Dispozitivelor Medicale este o autoritate administrativă, abilitată cu competenţe de reglementare şi supraveghere în domeniul medicamentului, activităţii farmaceutice şi dispozitivelor medicale.

OGYÉI
Országos Gyógyszerészeti Intézet
Országos Gyógyszerészeti és Élelmezés-egészségügyi Intézet (OGYÉI) látja el a gyógyszerészeti államigazgatási feladatokat, valamint egyes feladatok tekintetében egészségügyi államigazgatási szervként is kijelölték.

AIFA
Agenzia Italiana del Farmaco
L’Agenzia Italiana del Farmaco è l’autorità nazionale competente per l’attività regolatoria dei farmaci in Italia. E' un Ente pubblico che opera in autonomia, trasparenza e economicità, sotto la direzione del Ministero della Salute e la vigilanza del Ministero della Salute e del Ministero dell'Economia e Finanze.

AEMPS
Agencia Española de Medicamentos y Productos Sanitarios
La AEMPS, como agencia estatal adscrita al Ministerio de Sanidad, Consumo y Bienestar Social, es responsable de garantizar a la sociedad, desde la perspectiva de servicio público, la calidad, seguridad, eficacia y correcta información de los medicamentos y productos sanitarios, desde su investigación hasta su utilización, en interés de la protección y promoción de la salud de las personas, de la sanidad animal y el medio ambiente.

INFARMED
Autoridade Nacional do Medicamento e Produtos de Saúde
O INFARMED - Autoridade Nacional do Medicamento e Produtos de Saúde, I. P., abreviadamente designado por Infarmed, é um instituto público de regime especial, nos termos da lei, integrado na administração indireta do Estado, dotado de autonomia administrativa, financeira e património próprio.

ANSM
Agence nationale de sécurité du médicament et des produits de santé
L’Agence nationale de sécurité du médicament et des produits de santé (ANSM) a été créée par la loi du 29 décembre 2011 relative au renforcement de la sécurité sanitaire des médicaments et des produits de santé.
科学委員会
Prof. Lodovico Perletti
Pediatrician
Primario Pediatra dell' Ospedale di Melegnano. Iscritto alla Società Italiana di Pediatria. Consulente del Ministero della Salute per le Politiche Sanitarie ed i servizi materno-infantili.
Dott. Pietro Cifarelli
Dentist
Il Dott. Cifarelli si è laureato in Odontoiatria e Protesi Dentaria con lode nel 1993 presso l'Università degli Studi di Milano. Dopo la laurea frequenta numerosi corsi di approfondimento e aggiornamento odontostomatologico con particolare propensione alla terapia parodontale, endodontica e chirurgia implantologica. Iscritto alla Società Italiana di Implantologia Osteointegrata (SIO). Albo Provinciale degli Odontoiatri di Milano n. 1898 iscritto il 14/02/94
Dott. Marcello Mariani
Regulatory Affairs
Experienced Sales Director with a demonstrated history of working in the health wellness and fitness industry. Strong information technology professional skilled in Negotiation, Business Planning, Sales, Regulatory Affairs, Sales Operations, and Sales Management.
Dott. Marco Selle
Pharmacist
With a degree in pharmacy from the University of Milan, Dott. Selle in an experienced pharmacist with strong expertise in alternative and homeopatic medicine.
パートナー
ECHAlliance
European Connected Health Alliance
欧州の接続ヘルス·アライアンスは、ヨーロッパの両端に接続し、mHealth市場の発展と実践のためにリーダーシップを発揮します。ECHAllianceとmyHealthboxは、接続された健康への取り組みを促進するために戦略的なレベルで連携して動作します。
AIPIA
Active & Intelligent Packaging Association
AIPIA mission is to promote the commercial applications of Active & Intelligent Packaging solutions. AIPIA believes that implementation of new technologies in packaging is key to growth, secures protection, enhance efficiency, reduce waste and gain control in sales at end-user level.
Pharmatune
Healthcare products eCommerce (Greece)
Pharmatune is a platform, that uses a unique technology for real–time synchronisation with Pharmacies and their stock. Users will be able to find the pharmacies that stock their products along with real-time feedback on the availability of those products from anywhere, at anytime.
Vresgiatro
Medical network (Greece)
Vresgiatro is the only online medical network in Greece providing holistic health solutions to both patients and doctors.Patients can find doctors and schedule their visits via the Vresgiatro platform.They are being offered a wide range of options based on the doctor specialty, equipment and proximity as well as on their medical insurance.
BugiardinoOnline.com
Patients information leaflets search engine (Italy)
A fast search engine for patient information leaflets (PILs) and Summary of Product Characteristics (RCP) documents for all pharmaceutical products available in Italy. This service uses our web API to provide search and document view capabilities.
Cardiotool
Cardiology information portal (Italy)
An healthcare portal for patients and professionals about cardiology. CardioTool is a unique project with the goal of becoming a daily working tool for every GP, it offers the ability to quickly find in a single site all the information needed to solve the problems of clinical practice CV, even during a visit with the patient.
PIF Online
Patient Information Forum (UK)
The Patient Information Forum (PIF) - www.pifonline.org.uk - is a not-for-profit, membership organisation and network for people working in, and involved with, healthcare information and support. We are committed to improving the healthcare experience for patients and the public by helping individuals and organisations to deliver high-quality, evidence-based, accessible information and support, so that everyone can understand their care, and make informed decisions about their health and wellbeing choice.
Fondazione Rava
Fondazione Rava (Italy)
La Fondazione Francesca Rava aiuta l’infanzia in condizioni di disagio in Italia e nel mondo. Rappresenta in Italia NPH (Nuestros Pequenos Hermanos) presente dal 1954 in 9 paesi dell’America Latina con Case famiglia e ospedali e ad Haiti in particolare, da oltre 30 anni, con 2 Case in cui vivono 600 bambini, 3 ospedali, 2 centri per bambini disabili, 35 scuole. La Fondazione rappresenta inoltre in Italia la Fondazione St. Luc di Haiti. In Italia aiuta invece nelle emergenze quali la lotta contro l’abbandono neonatale, l’aiuto ai bambini in povertà sanitaria e la consegna di scuole per i bambini del Centro Italia colpiti dal terremoto.
Pharmfair.com
Drugs information (Singapore)
Pharmfair.com is founded and actively managed by a group of registered pharmacists. They aim to provide fast and accurate access to information on medicinal products licensed for sales in Singapore.
MMI
Medizinische Medien Informations (Germany)
Die Medizinische Medien Informations GmbH (MMI) bietet umfassende und unabhängig aufbereitete Arzneimitteldaten für Ärzte, Kliniken, Pflegeheime, Apotheken, Behörden und Healthcare-Unternehmen.
e-PIL
electronic Patient Information Leaflets (Germany)
One of the largest databases of digital Patient Leaflets and SmPC documents.
認証
HONcode
Standard for trustworthy health
This site complies with the HONcode standard for trustworthy health information:
WIS
Acreditación de webs de interés sanitario
Con este sello, el Comité Evaluador de PortalesMedicos.com, que ya supervisa el resto de las secciones de nuestro medio, distinguirá, entre todos aquellos que lo soliciten, a aquellos que puedan resultar de interés para la población general interesada por la Salud o para los profesionales sanitarios.